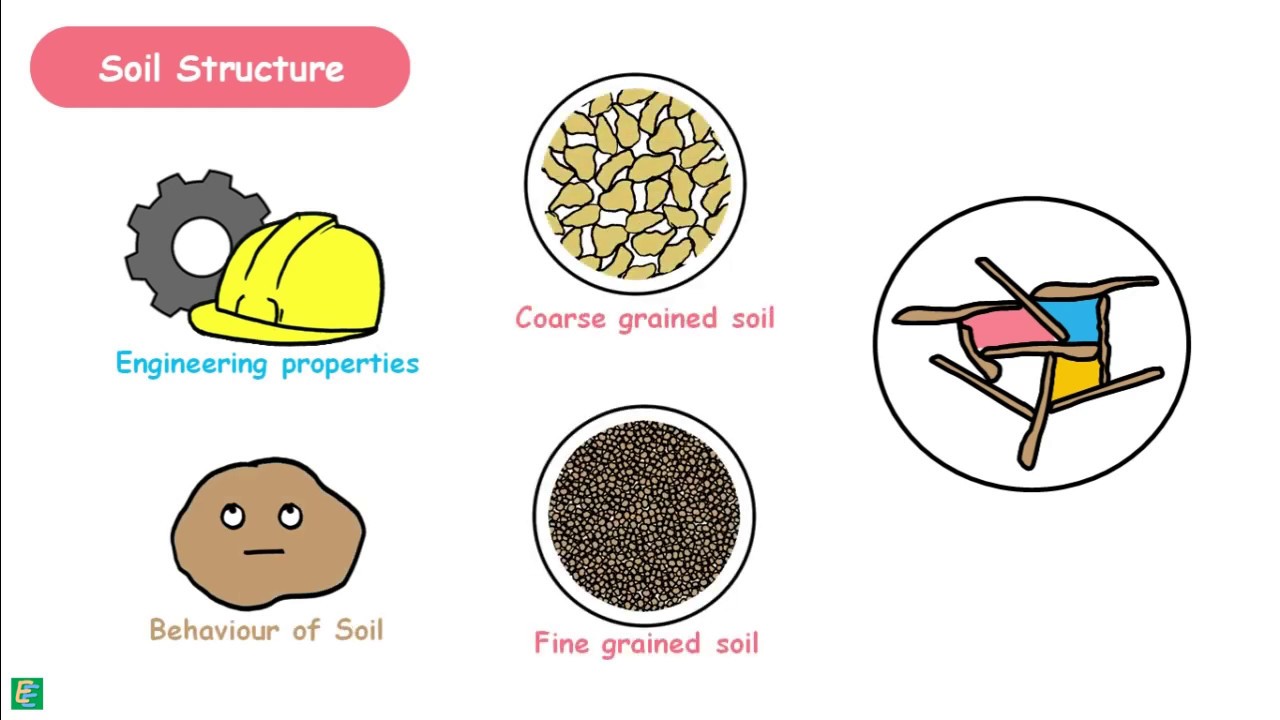
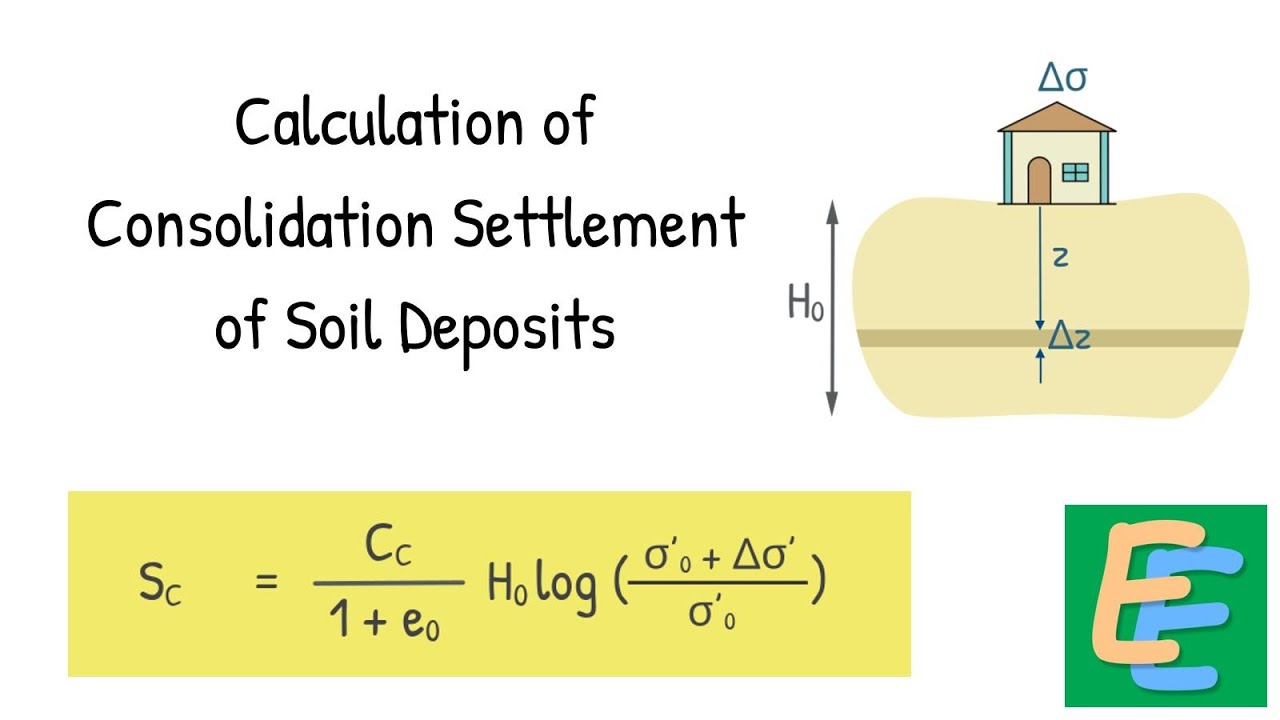
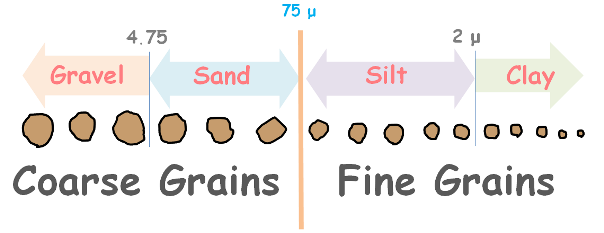
Soils are generally divided on the basis of their particle size into coarse grained and fine-grained soils. These are further divided into subcategories.
All these soils are made up of different types of rock minerals.
 Gravel, sand and silt soils are generally made up of primary rock-forming minerals like Quartz and Feldspar. The engineering properties (cohesion, permeability, compressibility etc.) of such soils do not depend upon the nature of the mineral present because these minerals are strong and electrically inert.
Gravel, sand and silt soils are generally made up of primary rock-forming minerals like Quartz and Feldspar. The engineering properties (cohesion, permeability, compressibility etc.) of such soils do not depend upon the nature of the mineral present because these minerals are strong and electrically inert.
Clay soils, on the other hand, are made up of minerals which are evolved from the chemical weathering of primary rock-forming minerals.
The change in contents and structures that occur in rocks due to physical, chemical and biological processes is called weathering.
 Clay mineral particles are tiny crystalline substances, very small in size and flaky in shape. These particles are microscopic.
Clay mineral particles are tiny crystalline substances, very small in size and flaky in shape. These particles are microscopic.
From the term clay we usually think of clay soil which is composed of clay minerals and exhibit properties like plasticity and cohesion. But not all clay sized particles are clay particles.
We can grind a rock into very fine particles of size smaller than 2 micron. These particles will be clay sized but not clay soil particles. Soil made up of these particles won’t be able to exhibit plasticity characteristics because they lack clay minerals which impart these properties to the clay soil.

Clay minerals are separated from gravel, sand and silt because they carry electrical charges on their surface. Clay soil also creates significant engineering problems such as high volume increase when comes into the contact of water and volume decrease when dried. These volume changes create cracks in the soil which are harmful to heavy construction and roads.
So let’s discuss clay mineralogy.
 Almost all the clay minerals are made up of two fundamental crystal sheets joined through different bonds.
Almost all the clay minerals are made up of two fundamental crystal sheets joined through different bonds.
One is tetrahedral Sheet which is also called silica sheet
And another one is octahedral Sheet also called Alumina Sheet or Gibbsite Sheet.
Silica Sheet:
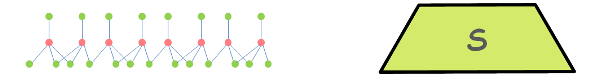
A Tetrahedral Sheet is made up of several Silica tetrahedral units combined together.
 One Silica Tetrahedral Unit consists of a silicon ion (Si4+) surrounded by four oxygen ions (O2-) forming a shape of tetrahedron. Silicon sits at the centre and oxygen ions sit at the tips of tetrahedron.
One Silica Tetrahedral Unit consists of a silicon ion (Si4+) surrounded by four oxygen ions (O2-) forming a shape of tetrahedron. Silicon sits at the centre and oxygen ions sit at the tips of tetrahedron.
Many such tetrahedral units combine to form the Silica Sheet.
Each oxygen ion at the base of the unit is common to two adjacent units. Hence two negative charge of each oxygen ion is shared by two units leaving one negative charge for one unit.

Oxygen ion sitting at the top does not share its charge with anyone. Central ion has plus four charge and base oxygens have -1 charge.

Hence in the sheet net charge per tetrahedral unit is -1.
(-2) + (+4) + {3x(-1)} = -1
This complex structure of silica sheet is symbolically represented as this.
Alumina Sheet or Gibbsite Sheet:
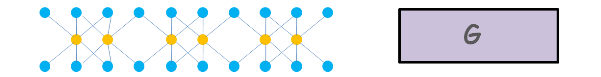
 A gibbsite sheet is made up of several Alumina octahedral units combined together.
A gibbsite sheet is made up of several Alumina octahedral units combined together.
One octahedral unit consists of six hydroxyls forming a configuration of an octahedron and having one aluminium ion at the centre.
Several such octahedral units combine to form a Gibbsite Sheet. It is represented by this symbol.
 If the central Aluminium ion in the octahedral is replaced by Magnesium ion, it results in formation of different crystal sheet called Brucite Sheet.
If the central Aluminium ion in the octahedral is replaced by Magnesium ion, it results in formation of different crystal sheet called Brucite Sheet.
This substitution of a metallic ion by other metallic ion of lower valence but of same physical size is called isomorphous substitution. This kind of substitution leads to formation of different soil mineral which has different physical properties.
Different clay minerals simply consist of these two basic sheets stacked together in certain unique fashion. Let’s discuss structure of few important clay minerals.
Kaolinite
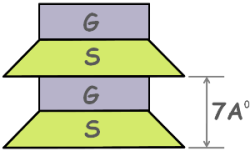 Kaolinite mineral is made up of silica and gibbsite sheets. In its basic structural unit these are stacked one over the other and tips of silica sheets are embedded in the gibbsite sheet. The thickness of such structural unit is about 7 Angstrom.
Kaolinite mineral is made up of silica and gibbsite sheets. In its basic structural unit these are stacked one over the other and tips of silica sheets are embedded in the gibbsite sheet. The thickness of such structural unit is about 7 Angstrom.
1 Angstrom is equal to 10-10 m or 1 m is equal to 1010 Angstrom.
As this mineral is formed by stacking of one layer of each sheet it is sometimes called a 1:1 clay mineral.
 Kaolinite mineral is formed by staking one over the other such several basic units. This unit extends indefinitely in other dimensions. These structural units join together by hydrogen bond between hydroxyls of alumina sheet and oxygen of silica sheet.
Kaolinite mineral is formed by staking one over the other such several basic units. This unit extends indefinitely in other dimensions. These structural units join together by hydrogen bond between hydroxyls of alumina sheet and oxygen of silica sheet.
As the hydrogen bond is sufficiently strong, the kaolinite mineral is stable and water cannot easily enter between the structural units and cause expansion.
kaolinite is the least active of all clay minerals.
Montmorillonite
 Another important clay mineral is montmorillonite.
Another important clay mineral is montmorillonite.
The basic structural unit of montmorillonite consists of a gibbsite sheet sandwiched between two silica sheets to form a single layer. The thickness of this layer is about 10 A and clearly it is a 2:1 mineral.
Montmorillonite mineral is formed by many such structural units joined together by Van der Waals force which is a very weak force when compared to hydrogen bond.
 Clay soils that attract more water have more plasticity, more swelling or shrinkage.
Clay soils that attract more water have more plasticity, more swelling or shrinkage.
Water molecules are dipolar and negatively charged surface of silica sheet attracts these molecules in the space between two structural units causing the layers of the mineral to be further separated which results in the expansion of the mineral.
That is why soils containing clay mineral montmorillonite exhibit high volume change. They swell as the water enters into the structure and shrink as the water is removed because of some reason.
Its excessive swelling capacity may seriously endanger the stability of overlying structures.
In India, clay soils containing montmorillonite mineral are commonly known as black cotton soils. It covers about country’s 20 percent area in the states of Andhra Pradesh, Karnataka, Madhya Pradesh, Maharashtra and Uttar Pradesh.
Illite
Then there is another important clay mineral called illite.
Its basic structural unit is similar to that of montmorillonite, so it is also a 2:1 mineral and layer thickness is also about 10 A.

In this mineral there is isomorphous substitution of silicon ions in silica sheet by aluminium ion. Also the potassium ions occupy the space between different structural units and do not allow water to take its place.
Potassium ion bonds the two layers together more firmly which was not the case in the montmorillonite. Therefore, illite does not swell as much in the presence of water as montmorillonite, but still it does much more than kaolinite.
The properties of illite mineral are somewhat lies between that of kaolinite and montmorillonite mineral.
Soils containing illite swell more than that of soil containing kaolinite but less than that of soil containing montmorillonite.