From the bridges you cross every day…
To the engines that power your vehicles…
From the satellites orbiting far above…
To the tiny microchips powering the phone in your hand —

there is one invisible yet powerful phenomenon, that quietly influences how all these things are designed and built, and how they survive the test of time: Thermal Expansion.
It’s an effect that can make materials grow, shrink, bend, crack — or fail. All because of a simple change in temperature.
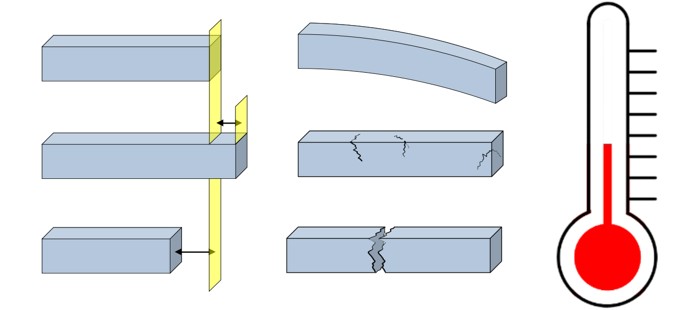
And if we ignore how temperature changes affect a design, the consequences aren’t just minor flaws — they can become dangerous structural problems. A material might crack, a part might jam, or an entire system could fail.
That’s why every engineer must understand it — not just as a formula, but as a real-world factor that affects every structure and system exposed to temperature changes.
So, what exactly is happening inside a material when temperature changes? Why does it expand, or sometimes shrink?
The science begins at the atomic level. Every solid, liquid, and gas is made of tiny atoms that are never truly still — they’re always vibrating. Which means everything around us — from the steel beams in a skyscraper to the glass in a window — is alive with this constant, invisible motion.

When the temperature rises — maybe from the warmth of the sun or the heat of a working engine — we’re essentially giving those atoms more energy. They start vibrating faster and with greater intensity, pushing their neighbours slightly farther apart. This tiny, invisible dance of atoms creates a cumulative effect: the material expands. Its length, width, and volume all increase.
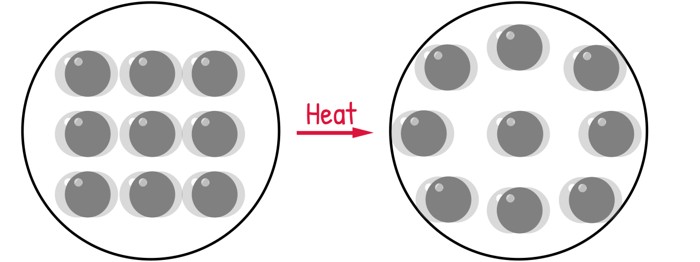
And this is what we call Thermal Expansion — a change in a material’s dimensions caused by a change in temperature.
Although the word “expansion” suggests only increase, but it is the conventional term used to describe both the expansion and the contraction that happens with temperature changes.
So, when the temperature drops, vibrations slow down, the particles settle a little closer together, and the material shrinks.
This change is usually tiny — often just fractions of a millimetre for everyday objects — but in engineering, even that much can mean the difference between a perfect fit and a jammed mechanism… or between a stable bridge and a cracked joint.
But is there a way to know exactly how much a temperature change will increase the length of a material?
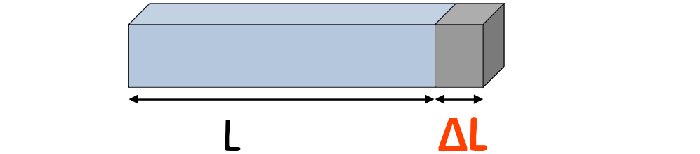
Yes. This is the formula for Thermal expansion in the material
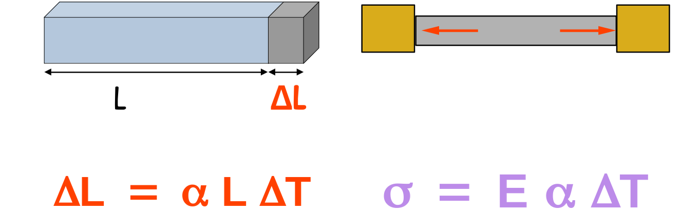
ΔL=α L ΔT
• ΔL is the change in length.
• α is the coefficient of linear expansion, a property of the material.
• L is the original length.
• ΔT is the change in temperature.
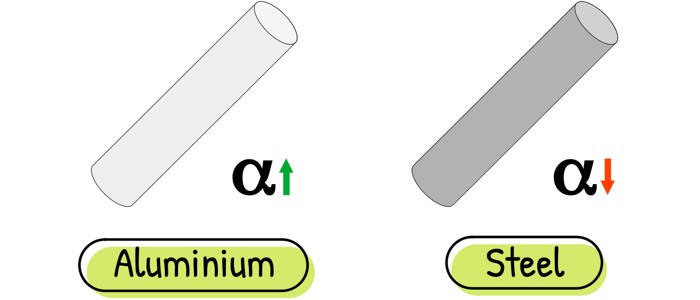
This simple-looking equation tells us that expansion (ΔL) depends on just three things: how long the material is (L), how much the temperature changes (ΔT), and a unique property called the coefficient of linear expansion (α). This coefficient is like a material’s “willingness” to expand. Think of aluminum: eager to stretch. Steel? More reluctant.
Technically, this is called linear expansion, because the material is expanding in one direction, along its length. But in reality, when a material is heated, it doesn’t just grow longer — it grows in all directions.
So, When its entire surface area increases, we call it Area Expansion / Superficial Expansion.
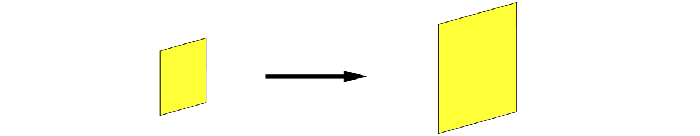
When its entire volume changes, we call it Volumetric Expansion / Cubical Expansion.
In most engineering applications — like beams, rods, or rails — we usually deal with linear expansion. For objects like window glass, areal expansion becomes important. And in cases like tanks, containers, or fluids, volumetric expansion is the crucial factor.
- Linear Expansion (1D)
ΔL=α L ΔT - Area Expansion (2D)
ΔA=2α A ΔT - Volumetric Expansion (3D)
ΔV=3α V ΔT
Each of these uses a corresponding coefficient of thermal expansion (α, 2α, 3α)— unique to the material and the type of expansion.
So, once engineers know how much a material can grow, they design deliberate, calculated gaps in pavements and install expansion joints in bridges, giving the material enough room to expand and contract freely as temperatures rise and fall.
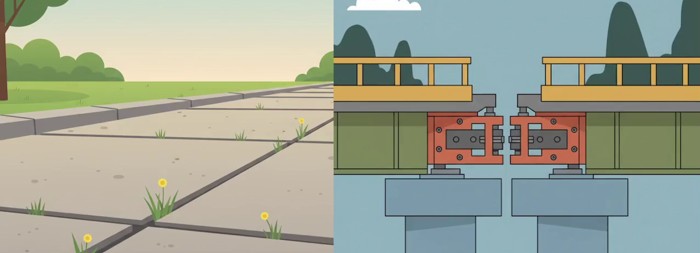
Now, here’s where it gets interesting — and dangerous.
What happens if we don’t let the material expand? What if that steel beam in a bridge is locked in place, with no gaps left to grow?
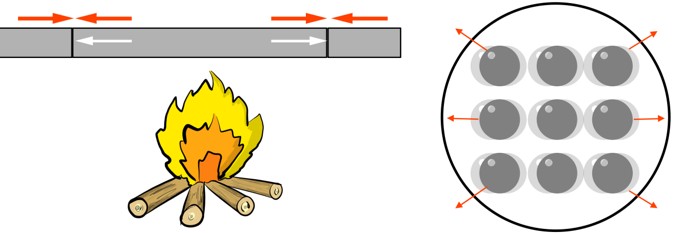
The atoms still try to push outwards, but the unyielding constraints are pushing back. The material is trapped. As the temperature rises, an invisible internal pressure builds up, a silent struggle between the material’s desire to expand and the forces holding it in place. This powerful internal pressure is what we call Thermal stress.
If that stress becomes too great, the material will eventually give way — often with catastrophic results. This is the invisible battle engineers face every day. It’s the reason why railway tracks buckle in the summer sun, why sidewalks have those deliberate gaps, and why a hot glass can suddenly shatter when cold water is poured into it.
But there are certain designs and situations, where we can’t leave gaps. Think of the engine of your car working for hours when you take it on a long trip. It gets incredibly hot, causing its parts to expand — yet there are no expansion gaps in its design. In such cases, the stresses that develop must remain less than what the material can safely handle.
So, how do engineers calculate the stress that builds up in a material when its expansion is restricted?
Here comes the second formula — used in designs where leaving a gap isn’t an option. This formula calculates the invisible thermal stress (σ) that builds up when a material is constrained:
σ = E α ΔT
• σ is the thermal stress.
• E is Young’s modulus, which measures the material’s stiffness.
• α is the coefficient of linear expansion.
• ΔT is the change in temperature.
This stress (σ) depends on three key factors: the material’s stiffness, known as Young’s modulus (E); its “willingness” to expand (α); and, once again, the change in temperature (ΔT).
This formula reveals the invisible pressure that builds inside a restrained material. It tells engineers whether a welded pipe will survive the summer heat, or whether a glass window might shatter the instant cold rain splashes against its hot surface.
In practice, both formulas work hand in hand. One predicts movement, the other warns of the forces when that movement is restricted. Together, they turn invisible atomic vibrations into numbers, a language that engineers can use to design with — and lives can depend on.
This simple understanding of thermal stress fundamentally changes how we build our world. The applications of these principles extend far beyond bridges and sidewalks. They are the invisible rules governing our engineered world, from the massive scale of industrial machinery to the microscopic world of electronics.
Consider the steam pipes in a power plant, which are often rigidly fixed at key points to manage weight and pressure. However, these pipes carry extremely hot fluids, and if they didn’t have room to expand, the relentless heat would build up enough thermal stress to warp or rupture them. But since these are continuous pipes, a simple gap won’t work. Instead, engineers incorporate a brilliant solution: they install what is called an expansion loop — those deliberate U-shaped bends in the piping that provide a flexible section to absorb the thermal movement and protect the entire system.
The same challenge is faced in engines, turbines, and industrial machinery. Every part must have enough freedom to expand and contract without jamming or breaking under the immense heat and pressure.
And the challenge gets even more precise. On the tiny scale of microchips and circuit boards, engineers must account for minuscule expansions in materials. Without this understanding, repeated heating and cooling cycles can cause invisible cracks, solder joint failures, or warped boards that lead to device failure.

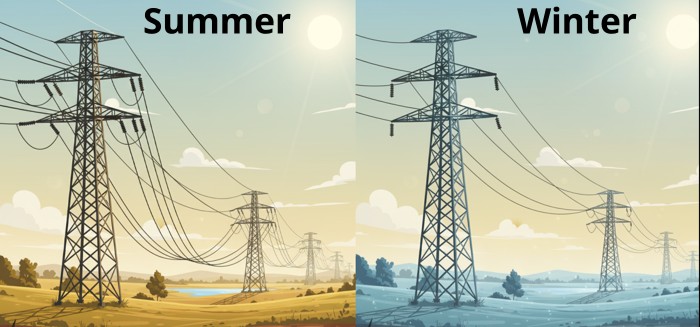
But you don’t need to be an engineer to see this in action. You might have noticed how overhead electrical power lines seem to sag more on a hot summer day and become much tighter on a chilly morning. This is thermal expansion happening right above your head.
Whether you’re building a skyscraper, laying a pipeline, or designing a spacecraft, one truth remains: every material expands when heated and contracts when cooled. Accounting for this invisible dance of expansion and contraction is what keeps our world functional—and safe.
And now I’d love to hear from you: Have you ever noticed a sidewalk with those deliberate gaps, or seen a power line sagging in the heat? Or have you seen a more dramatic example of thermal failure? Share your observations in the comments below!